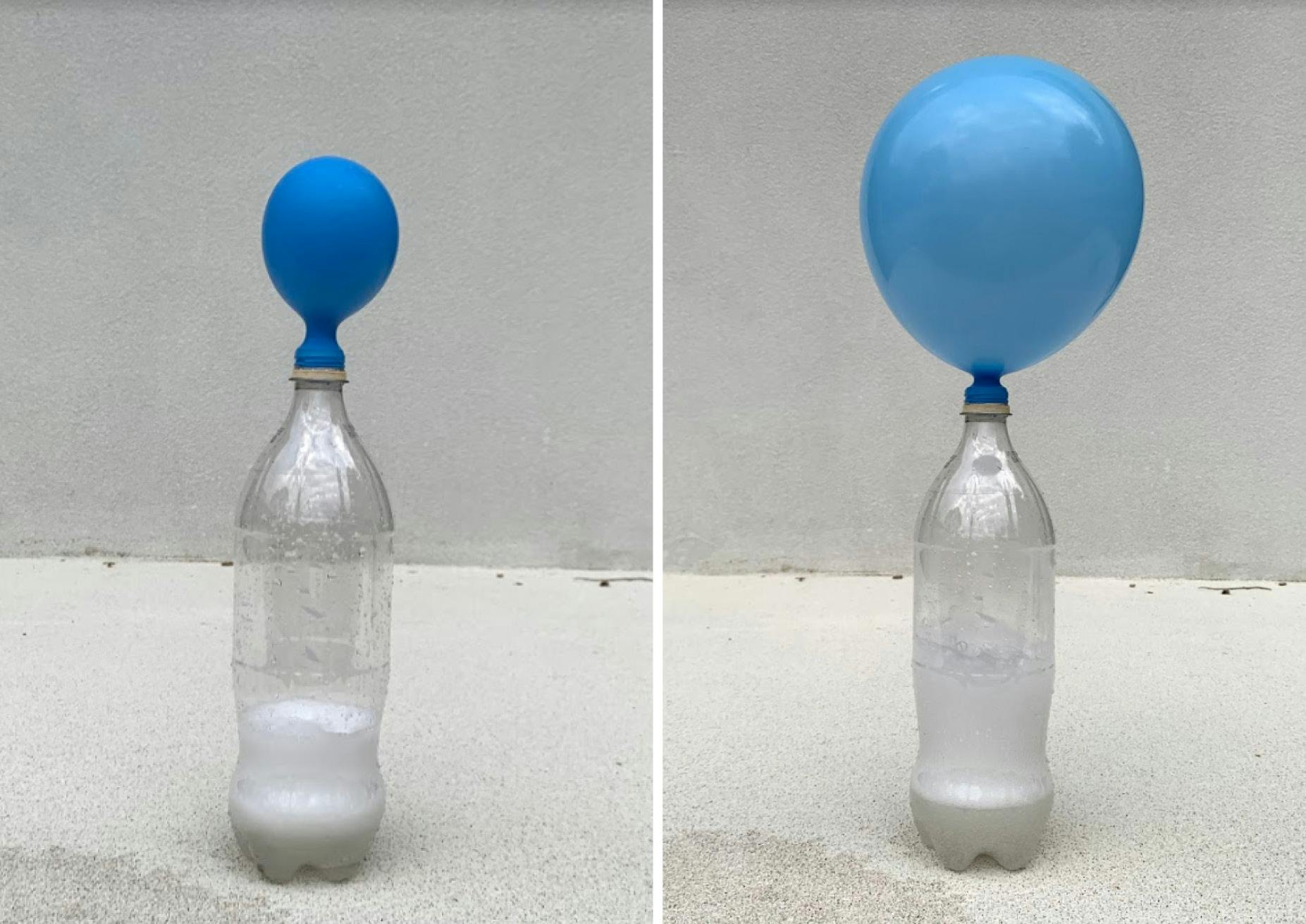
Baking soda and vinegar reaction inflate a gas balloon You can inflate a gas balloon without having to blow it itself First add some vinegar into the bottle Then fill the balloon with baking soda using the funnel Now the balloon is put gently on the neck Make sure that the balloon is firmly seated on the neckThis lab demonstrates the reactivity of two household cooking items, baking soda and vinegar Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid These 2 components react in solution to form carbon dioxide, water, and sodium acetate as shown in the chemical reaction belowWhen the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate Carbon dioxide is
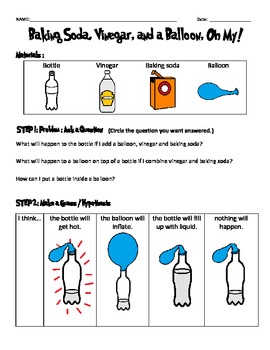
Baking Soda Vinegar And A Balloon Oh My Scientific Method Data Sheet
Baking soda and vinegar reaction fill balloon
Baking soda and vinegar reaction fill balloon-1 day ago When vinegar and baking soda mix, they create the gas carbon dioxide and water The carbon dioxide has no where to go, but into the balloon blowing it up But you should have found that the balloon at room temperature may have been slightly faster in blowing up your balloonOne person to hold the balloon open and the other person to put the baking soda inside of the balloon) Pour the vinegar into the bottle Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar




Balloon Baking Soda Vinegar Kids Science
Fun investigation into how adding more vinegar changes the baking soda and vinegar reaction Blow up balloon with baking soda and vinegar When vinegar is mixed with baking soda, a double replacement reaction takes place The end result is carbon dioxide but behind the scenes, there is more than one reaction taking place Put a tablespoon of backing soda on to the center of a paper towelWhen vinegar and baking soda mix, an endothermic reaction occurs This produces carbon dioxide which inflates the balloon The endothermic chemical reaction will also lower the temperature in the area surrounding the vinegar and baking soda so significantly that you will be able to easily notice the change in temperature
Instructions Use the funnel to add the 1/3 cup of baking soda into the balloon Twist the neck of the balloon a few times to keep the baking soda from spilling out and set the balloon aside Rinse the funnel and then use it to add the 1 cup of vinegar to the bottle Next, carefully stretch the Balloons Funnels Measuring spoon Start by putting the funnel into the balloon This will make it much easier to get the baking soda inside Add baking soda We added about 4 teaspoons to each balloon To get your balloons a bit bigger than ours, you could add 1 or 2 teaspoons more Next, use a funnel to pour some vinegar into your bottlesSet up a balloon baking soda and vinegar science experiment for kids Blow up balloons with a chemical reaction between baking soda and vinegar Fun science
Henry was getting a kick out of the experiment and loved watching it overflow the cupGrab Baking Soda, Vinegar and a balloon and you're set to create an amazing gas called Carbon Diox About Press Copyright Contact us Creators Advertise Developers TermsMost of the coolest and easiest science experiments happen thanks to the amazing chemical reaction between baking soda and vinegar So we have compiled a list of some of the best vinegar and baking soda experiments right here for you and your kids to enjoy!




Vinegar And Baking Soda Balloon




Baking Soda Vinegar And A Balloon Oh My Scientific Method Data Sheet
The baking soda will quickly react with the vinegar in the flask, creating carbon dioxide gas as one of its products, causing the balloon to quickly inflate After the reaction is complete the balloon will remain inflate You can pop the balloon with a tack if you wish to confirm with the students that the balloon was filled with gasUsing the funnel, add the baking soda to each balloon (two people may be needed for this;The best part of these vinegar and baking soda experiments is how easy, entertaining, and educational they are




Blowing Up A Balloon With Baking Soda And Vinegar Thriftyfun




Fun Science Experiments Baking Soda And Vinegar Balloon Experiment
Balloon Blowup AT A GLANCE In this activity, we will use simple materials to generate static electricity and explore its behavior STUDENTS WILL BE ABLE TO Use baking soda and vinegar to create chemical reaction that gives off gas as a product BACKGROUND INFORMATION Baking soda is a chemical called sodium bicarbonate and it reacts with Balloon STEM Activity Like our bottle rockets, in this activity we are capturing the CO2 gases that result from a baking soda and vinegar reaction Using our STEM skills we tested different ratios to see how it affects the inflating of our balloons We have done this experiment for Groundhog Day and Halloween When baking soda is mixed with vinegar, something new is formed The mixture quickly foams up with carbon dioxide gas If enough vinegar is used, all of the baking soda can be made to react and disappear into the vinegar solution Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate




Blowing Up A Balloon With Baking Soda And Vinegar Cool




Vinegar And Baking Soda Balloon Experiment Happy Brown House
When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda The result of this initial reaction is two new chemicals carbonic acid and sodium acetate The second reaction is a decomposition reactionIt is a showstopper experiment for kids (See more of my STEM projects for kids) How to Do the Baking Soda and Vinegar Balloon Experiment Supplies You Will Need for the ExperimentVinegar & Baking Soda Experiment by HashtagScienceThirteen 1 $300 Word Document File In this experiment, students will combine vinegar and baking soda in a water bottle to create a chemical reaction that inflates a balloon!




Baymax S Low Battery Baking Soda And Vinegar Experiment




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do
We could have kept going with this all afternoon!Pour two teaspoons of baking soda into your balloon, and pour half a cup of acetic acid into the bottle An intense reaction will begin, releasing CO2 and making the balloon inflate If the reaction is too weak to inflate the balloon, add more vinegar and baking soda , but don't shake the solutionPour two teaspoons of baking soda into your balloon, and pour half a cup of acetic acid into the bottle An intense reaction will begin, releasing CO2 and making the balloon inflate If the reaction is too weak to inflate the balloon, add more vinegar and baking soda , but don't shake the solution
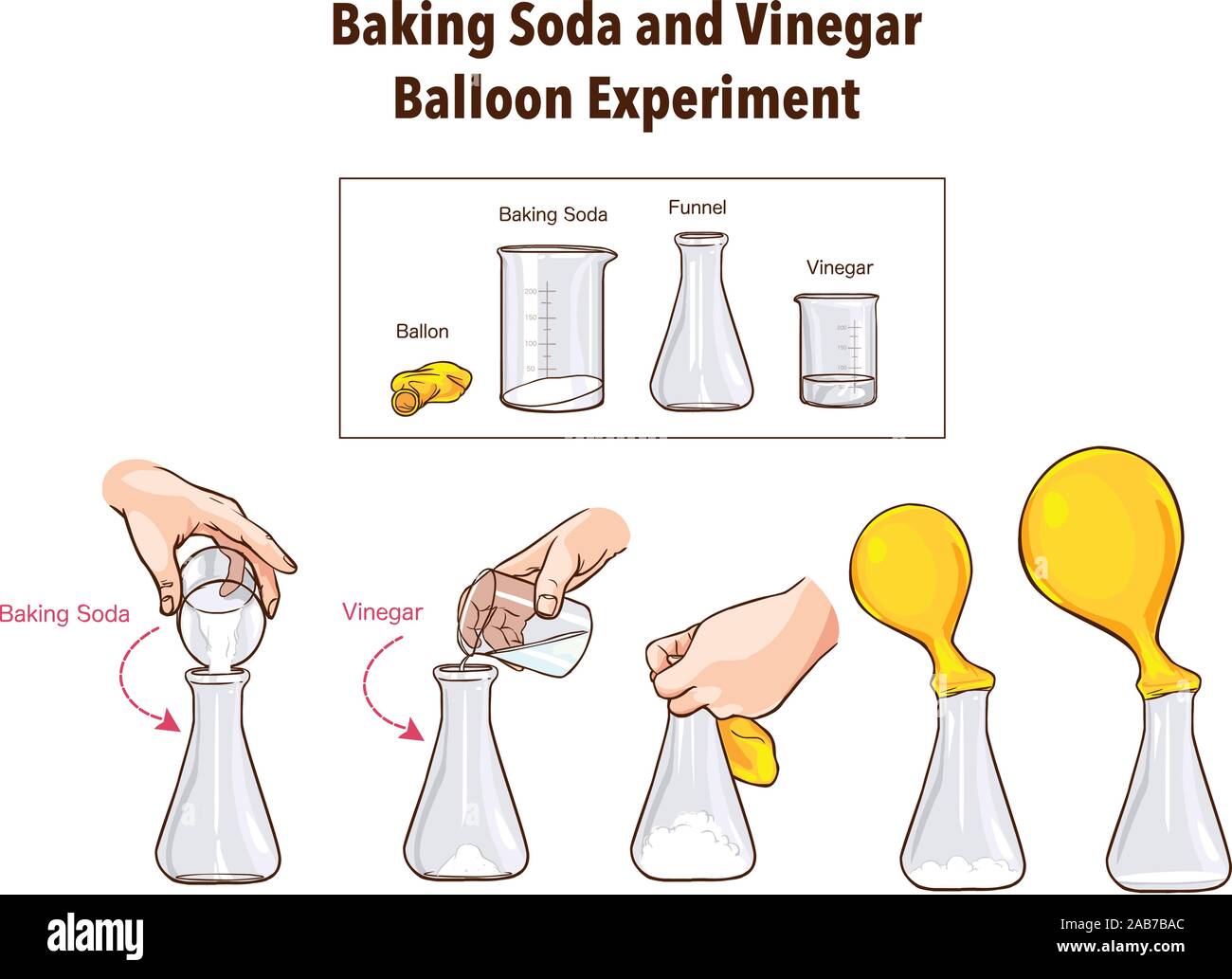



Baking Soda And Vinegar Balloon Experiment Science Stock Vector Image Art Alamy




States Of Matter Balloon Inflation Experiment Baking Soda Vinegar
That lift is the gas produced from the two ingredients is carbon dioxide or CO2 Instructions Stretch the opening of the balloon over the end of the funnel Pour about 1/3 cup of baking soda into the funnel and Rinse all the baking soda off the funnel, and then use the funnel to pour the vinegar into a water bottle Gently stretch the opening of the balloonWhen vinegar and baking soda are mixed together they create what is called a chemical reaction A gas is formed (carbon dioxide) which takes up more space than the vinegar and baking soda That is why the balloon inflates Other points to share if doing this experiment with older children The baking soda is a base while the vinegar is an acid




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




At Home Lesson Breathless Balloons With Edie S Experiments Penguin Books Australia
The reaction between the baking soda and vinegar cause the balloon to inflate all on its own!Step 3 Pour 2 or 3 teaspoons of vinegar to the water bottle using the funnel Step 4 Attach the balloon to the top of the water bottle, being careful to not let any of the baking soda slip in until you are ready Step 5 Quickly tip the vinegar so it mixes in with the baking soda Step 6 Watch as your balloon blows up Baking soda and vinegar are both innocuous chemicals that most people keep around the house for cleaning and cooking purposes, but when you mix them together, the results can be exciting Baking soda is sodium bicarbonate, and when it mixes with the acetic acid in vinegar, the reaction produces carbon dioxide




Blow Up A Balloon With Baking Soda And Vinegar Frugal Fun For Boys And Girls




Baking Soda And Vinegar Balloon Experiment
Pour vinegar (1 – 2 dL is enough) Using a funnel, pour baking soda in the bottle (1 – 2 spoons) Close the bottle with the cork and turn it over so it stands on the launching pad If the pressure builds too fast, you can put baking soda in a piece of paper, put in the bottle, put the cork on and shake it so you start a reaction;First, fill the water bottle about 1/3 of the way full with white vinegar Next, put baking soda into the uninflated balloon, filling it about halfway Ideally, you'd have a funnel handy for this process but, because I didn't have one, I made one out of construction paper rolled up, and tape It did the trick!I use it as a review for exothermic and endothermic reactions, acids, and bases



1




Blow Up Balloon This Time With Baking Soda And Vinegar Science And Samosa
Fun Science Experiments // Baking Soda and Vinegar Balloon ExperimentIncludes BOTH US size and Australian sized files/spelling!This fun, simple science experiment is an excellent hands on cost effective way to show children the three different states of matter and chemical changes in action!This experiment explores the chemical reaction between baking soda and vinegar,The vinegar and the baking soda mix together to make an acidbase reaction The reaction creates carbon dioxide gas that bubbles up from the mixture The gas expands up and out of the bottle and inflates the balloon4 Sept 15 Why does baking soda and vinegar inflate a balloon?Once the vinegar and baking soda mix, the balloon will start to automatically inflate For those inquisitive minds who want to know how this happens, the vinegar is an acid and baking soda is a base When you mix an acid with a base, it causes a chemical reaction




Inflate A Balloon Career Girls



Science Experiments For Kids Blow Up A Balloon With Vinegar And Baking Soda
Adding baking soda to vinegar, the reaction is delayed, but then fizzes the same amount More vinegar is better A 12 to 1 ratio of vinegar to baking soda caused a fizzing explosion! One way is to pour a little vinegar into the balloon and then use a string or rubber band to seal off the little part of the balloon with the vinegar in it Then pour in a little baking soda into the rest of the balloon Now seal off the whole balloon by tying off its opening Then open up the seal between the tip with the vinegar and the part with the baking soda They should react, releasing CO 2 which will blow up the sealed balloon The first reaction is the acidbase reaction When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda The result of this initial reaction is two new chemicals carbonic acid and sodium acetate The second reaction is a decomposition reaction




Baking Soda And Vinegar Balloon Experiment For Kids Science Experiments Kids Kid Experiments Balloon Experiment



The Science Of Baking Soda Jerry James Stone
The products of baking soda and vinegar are water (H2O) carbon dioxide (CO2) The CO2 gas is what fills the balloon Surprising Notice how the balloon bounces at the endThe balloon filled with CO2 from the chemical reaction is heavier than the reaction products of breathing air or helium (the two ways we would normally blow up balloons) The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift! Baking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate




How To Blow Up A Balloon With Baking Soda And Vinegar 9 Steps




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download
Does vinegar and baking soda make helium?Pour the baking soda into the balloon using a funnel Measure 45 ml of vinegar and pour it into a water bottle Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon (The balloon will be flopped to one side) Lift the balloon up and pour the baking soda into the bottle of vinegar Repeat for each type of vinegar Baking Soda Vinegar "Baking soda is basic and vinegar is acidic," says Bock "When you put them together you get mostly water and sodium acetate But really, just mostly water" Plus, vinegar causes baking soda to foam up If stored in a




Balloon Baking Soda Vinegar Science Experiment For Kids Baking Soda Experiments Balloon Science Experiments Kid Experiments




Balloon Baking Soda Vinegar Science Experiment For Kids
The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions The reaction proceeds in two stepsWhat is the purpose of inflating a balloon with vinegar and baking soda?Baking soda Chemical name, sodium bicarbonate with formula NaHCO 3 Vinegar A dilute solution of acetic acid in water Acetic acid is also known as ethanoic acid, with the formula CH 3 COOH A beaker or jar The chemical reaction When baking soda is mixed with vinegar, something new is formed The mixture quickly foams up with carbon




Baking Soda And Vinegar Balloon Experiment Science Project Education Com




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do
Using the funnel, put the baking soda into the balloon Pour the vinegar in the bottle Now attach the balloon to the opening of the balloon in such a way that it fits closely, without leaving any gap Hold the balloon up so that the baking soda falls into the vinegar




How To Blow Up A Balloon With Baking Soda And Vinegar 9 Steps




Mama S Little Muse Science Activity Blowing Up A Balloon Using Baking Soda And Vinegar




Baking Soda Vinegar Balloon Experiment The Go To List




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity




Mom To 2 Posh Lil Divas Blow It Up Exploring Gas With Balloons Baking Soda Vinegar



1




Baking Soda And Vinegar Balloon Experiment Balloon Blowing Up 2 Happy Brown House
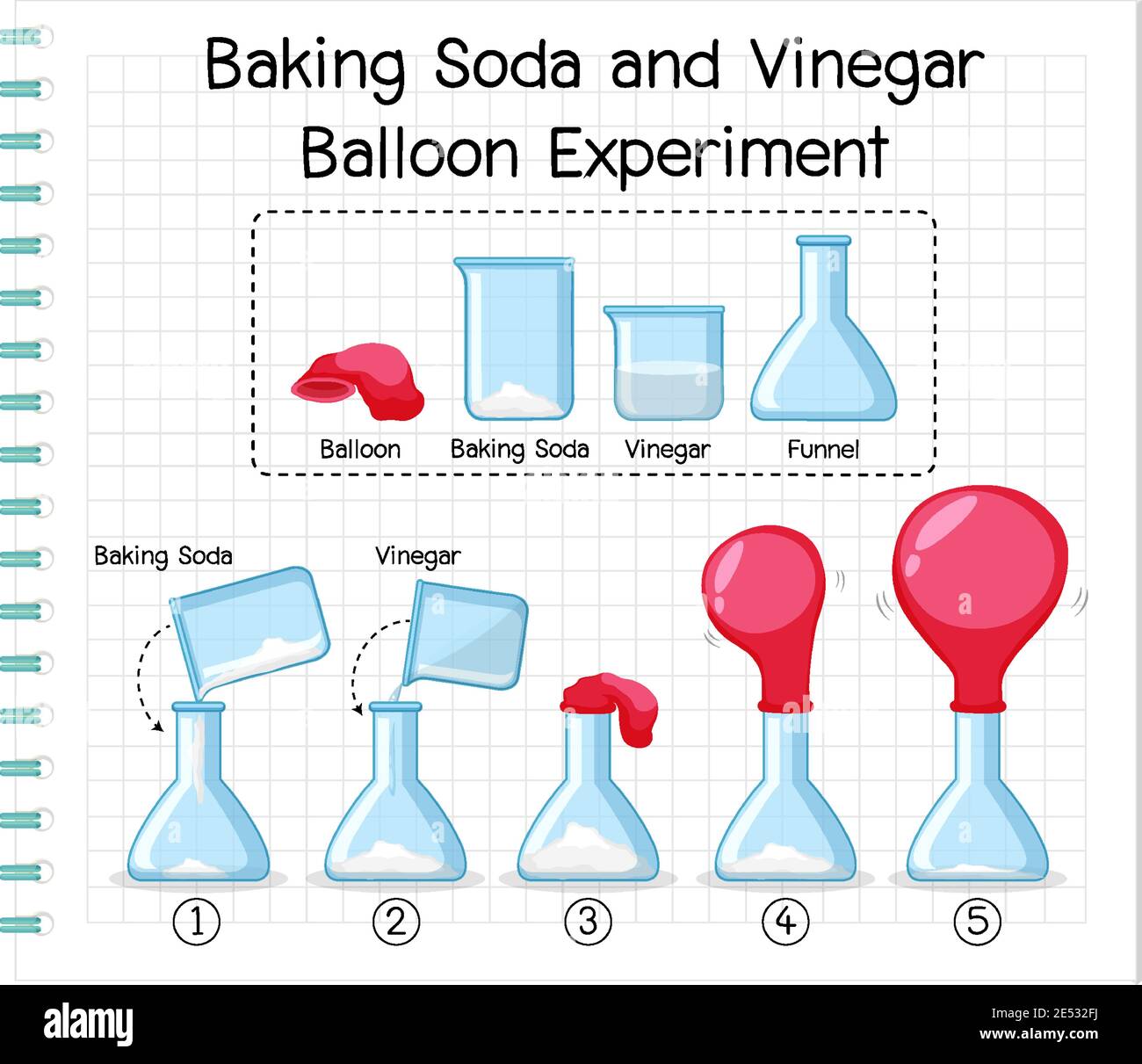



Science Experiment With Baking Soda And Vinegar Balloon Illustration Stock Vector Image Art Alamy




Baking Soda Vinegar Balloon Experiment Youtube




Baking Soda And Vinegar Balloon Experiment Science Royalty Free Cliparts Vectors And Stock Illustration Image




Balloon Baking Soda Vinegar Kids Science




Simple Science Experiment Chemical Reaction Brigitte Brulz




Blow Up Balloon This Time With Baking Soda And Vinegar Science And Samosa




Balloon Baking Soda Vinegar Science Experiment For Kids



Science Experiments For Kids Blow Up A Balloon With Vinegar And Baking Soda



1




Blowing Up Giant Balloon Baking Soda And Vinegar Experiment For Kids Youtube




How To Inflate Balloon With Vinegar And Baking Soda Science4fun




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Balloon Experiment Stem Activities Preschool Preschool Stem




Baking Soda And Vinegar S Reaction Perkins Elearning




Build A Fizz Inflator Sciencebob Com




Blowing Up Balloons With Baking Soda And Vinegar Pink Stripey Socks




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do




How To Blow Up A Balloon With Baking Soda And Vinegar At Home Science Experiments For Kids Jm Cremp S Adventure Blog




Research Question By Zaria Williams




Baking Soda And Vinegar Balloons




Baking Soda Vinegar And Our Atmosphere Experiment Wltx Com



1




Baking Soda And Vinegar Balloon Experiment




Balloon Baking Soda Vinegar Science Experiment For Kids




Balloon Baking Soda Vinegar Science Experiment For Kids




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity




Classroom Resources Inflating A Balloon With Chemistry ct




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity




Let S Play With Reactions




Baking Soda And Vinegar Balloon Experiment Balloon Blowing Up 1 Happy Brown House




How To Inflate A Balloon Using Baking Soda And Vinegar



At Home Lesson Breathless Balloons With Edie S Experiments Penguin Books Australia




Law Of Conservation Of Mass Lab




Baking Soda And Vinegar Balloons




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities




How To Blow Up A Balloon With Vinegar And Baking Soda Or Yeast




Scientist Squad Diy Baking Soda And Vinegar Science Experiments For Kids Blowing Balloon Valcano Balloon Magic Facebook
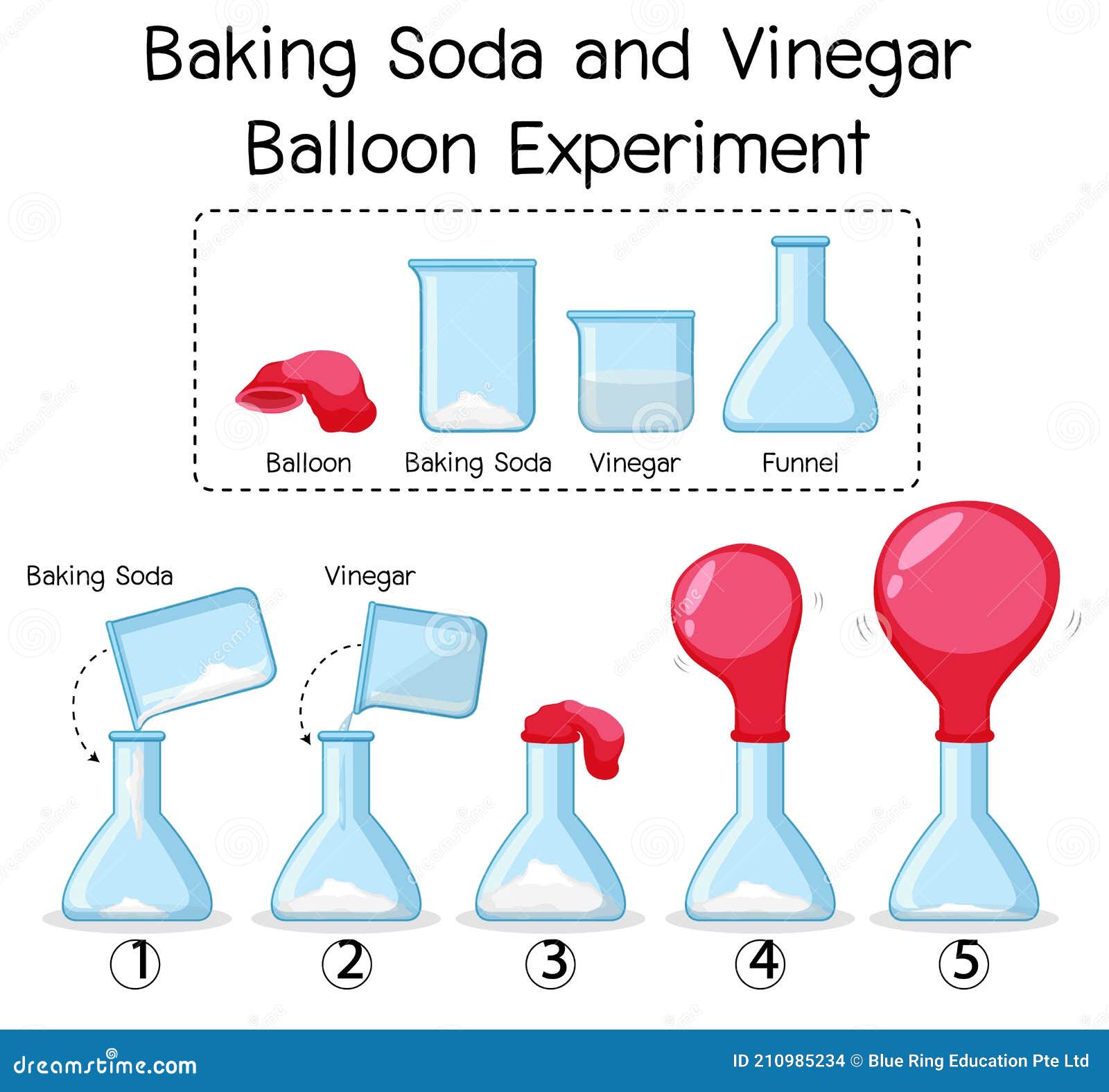



Science Experiment With Baking Soda And Vinegar Balloon Stock Vector Illustration Of Blank Object



Science With Children Baking Soda And Vinegar Experiments




Balloon Baking Soda Vinegar Kids Science




Baking Soda And Vinegar Balloons




Super Fun Experiment For Kids And Big Kids Blow Up Balloons With Vinegar Baking Sod Fun Experiments For Kids Blowing Up Balloons Balloon Science Experiments




Blow Up A Balloon With Baking Soda And Vinegar Frugal Fun For Boys And Girls




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




How To Fill A Balloon With Baking Soda Vinegar 5 Steps With Pictures Instructables




Mom To 2 Posh Lil Divas Blow It Up Exploring Gas With Balloons Baking Soda Vinegar




Vinegar And Baking Soda Balloon Activity Education Com




Baking Soda And Vinegar Balloons




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express




In This Experiment We Poured Vinegar Into The Flask And Baking Soda Into The Balloon We Wrapped The Balloon Around The Top Of The Flask And Carefully Ppt Download



Blowing Up Balloons With Co2 A Unique Hands On Science Night




Balloon Blow Up Science Experiment




Baking Soda And Vinegar Reaction Earth Day Science Experiment




Magic Balloon Inflation Baking Soda And Vinegar Experiment Midwestern Mama




Watercolor Balloons For Kids Learn Colors With Color Balloons Baking Soda And Vinegar Experiment Youtube




Chemical Reaction Experiment When Baking Soda Stock Photo Edit Now




Baking Soda And Vinegar Balloons




Baking Soda Vinegar And Our Atmosphere Experiment Wltx Com




Blowing Up A Balloon With Baking Soda And Vinegar Cool




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Baking Soda And Vinegar Balloon Experiment Youtube




Baking Soda And Vinegar Lesson Plans Worksheets




Balloon Science



Koch Newsroom Baking Soda Balloons



Baking Soda Vinegar Balloon Experiment Capturing Parenthood




Kid Science Hot Air Balloon Bottle Kix Cereal




Wait Weight Don T Tell Me Chemistry Earth Science Science Activity Exploratorium Teacher Institute Project




How To Blow Up A Balloon With Baking Soda And Vinegar Jm Cremp S Adventure Blog




Baking Soda And Vinegar Balloon Experiment Science Projects For Kids Educational Videos By Mocomi Youtube




Inflate A Balloon With Baking Soda And Vinegar Pbs Kids For Parents



0 件のコメント:
コメントを投稿